| Team Members: | Gemma Gearhart1, Shuai Jiang2, Thomas J. May3, and Jane Pan4 |
| Graduate Research Assistant: | Samuel Khuvis4 |
| Faculty Mentor: | Matthias K. Gobbert4 |
| Client: | Bradford E. Peercy4, Arthur Sherman5 |
1Division of Science, Mathematics and Computing, Bard College at Simon’s Rock,
2Department of Mathematics, Cornell University,
3Department of Mathematics, Virginia Polytechnic Institute and State University,
4Department of Mathematics and Statistics, University of Maryland, Baltimore County,
5Laboratory of Biological Modeling, National Institutes of Health

Team 4, from left to right: Jane Pan, Gemma Gearhart, Thomas J. May, Shuai Jiang
About the Team
Our team, Gemma Gearhart, Shuai Jiang, Thomas J. May, and Jane Pan, participated in the REU Site: Interdisciplinary Program in High Performance Computing located in the Department of Mathematics and Statistics at UMBC. Our project focused on pancreatic beta cells, which are responsible for the storage and production of insulin and therefore important in the study of Type I and Type II diabetes. Understanding the factors that affect insulin secretion and finding conditions that cause beta cells to behave in certain ways will allow researchers to gain a clearer approach in potentially treating diabetes. Through the tremendous assistance and feedback we obtained from our clients, Dr. Bradford Peercy from the Mathematics and Statistics Department at UMBC and Dr. Arthur Sherman from the Laboratory of Biological Modeling at NIH, our team ran successful simulations and produced useful results that allowed us to gain a better understanding of oscillation behaviors within beta cells. Additional guidance and support was provided by our faculty mentor, Dr. Matthias Gobbert, and our graduate research assistant, Samuel Khuvis.
Project Introduction
Diabetes mellitus is a group of diseases in which insulin resistance or insulin deficiency develops, causing high blood glucose. Pancreatic beta cells, the biological units responsible for the secretion of insulin, are located in islets of Langerhans. These cells exhibit oscillations in voltage and the concentrations of certain metabolites, including FBP and G6P, and can be ‘coupled’ to one another through intercellular connections called gap junctions. We simulated an islet of beta cells with an NxNxN cube in which each cell is characterized by a system of seven ordinary differential equations. By modifying and expanding previously developed Matlab code, we were able to observe the effects of metabolic coupling and mutated KATP channels on oscillations in computational islets and begin to describe the conditions that lead to their loss.
Classes of Metabolic Behavior
Oscillations in voltage and chemical concentrations within beta cells correspond to the rate of insulin production. When there are two heterogeneous cell types (with differing initial conditions), there are three main classes of metabolic behavior exhibited. These classes are pictured in figure 1 and are described as continued oscillation, asynchronous oscillation death, and synchronous oscillation death.

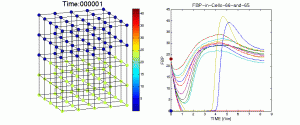
Figure 1Figure 2 is a visualization of changing concentrations of the metabolite FBP in a 125-cell islet. Concentrations are plotted by the given color scale in each cell for 50,000 time steps representing approximately 8.3 minutes. The figure on the left displays the line plots for FBP concentration in each cell of the islet and traces one cell of each of the two heterogeneous types.
Effect of Metabolic Coupling on Metabolite Concentrations
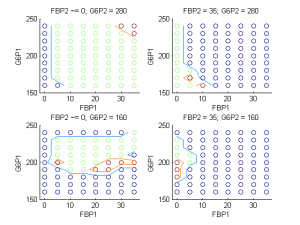
To test the ways in which oscillation behaviors can be changed, we coupled cells in our islet electrically and metabolically and observed the effect on metabolic behavior. We found that certain combinations of coupling strengths and initial conditions resulted in the loss of slow metabolic oscillations. Figure 3 displays the classes of metabolic behavior at varying initial concentrations of two metabolites in the two types of cells when all other initial conditions and parameters, including coupling strength, are kept constant. Blue regions correspond to continued oscillation, red regions correspond to synchronous oscillation death, and green regions correspond to asynchronous oscillation death.
Effect of Open KATP Channel Mutations on Voltage
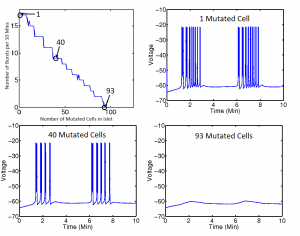
We also considered islets with varying proportions of mutated cells in which KATP channels are always open. For this portion of our project, we focused on the effects of the addition of mutated cells on electrical bursting. Figure 4 shows that the number of electrical bursts per cell in an electrically coupled 5x5x5 islet decreases as mutations are added, until 93 of the 125 cells, or 74 percent, are mutated. Once the number of mutated cells reach this threshold, bursting behavior disappears. The islet maintains constant coupling strengths and parameter values.
Conclusions
Figure 3 shows an example of how we can predict the solution of our system (via the class of metabolic behavior) given a set of coupling strengths and the initial conditions of our two metabolites. We can facilitate a shift from one solution to another by providing a perturbation sufficient to change the concentrations of the two metabolites to levels that result in a different solution. The bursting death threshold in cells with open channel mutations (for example, the 74 percent threshold in figure 4) is independent of coupling strengths and mutated cell arrangement within the islet, and may also be independent of islet size. However, it is not independent of a parameter that describes the glucokinase reaction rate of the cells. Further studies in this section of our project would include looking at effects of mutation on electrical bursting in islets with cells whose KATP channels are always closed, rather than always open. Our project provides the structure necessary for investigating this case in the future.
Links
Gemma Gearhart, Shuai Jiang, Thomas J. May, Jane Pan, Samuel Khuvis, Matthias K. Gobbert, Bradford E. Peercy, Arthur Sherman.Investigating Oscillation Loss in Computational Islets. Technical Report HPCF-2013-14, UMBC High Performance Computing Facility, University of Maryland, Baltimore County, 2013. Reprint in HPCF publications list
Poster presented at the Summer Undergraduate Research Fest (SURF)
Click here to view Team 1’s project
Click here to view Team 2’s project
Click here to view Team 3’s project