| Team Members: | Amanda M. Alexander1, Erin K. DeNardo2, Eric Frazier III3, Michael McCauley4, and Nicholas Rojina5 |
| Graduate Assistant: | Zana Coulibaly4 |
| Faculty Mentor: | Bradford E. Peercy4 |
| Client: | Leighton T. Izu7 |
1Department of Mathematics, Western Washington University,
2Department of Electrical and Systems Engineering, Washington University in St. Louis,
3Department of Mathematics and Computer Science, La Salle University,
4Department of Mathematics and Statistics, University of Maryland, Baltimore County,
5Department of Mathematics, University of North Carolina, Chapel Hill,
6Department of Pharmacology, University California, Davis
About the Team
Our team consists of Amanda Alexander, Erin DeNardo, Eric Frazier, Michael McCauley, and Nicholas Rojina. We worked with our graduate
assistant, Zana Coulibaly, and our faculty advisor, Bradford E. Peercy, on improving a mathematical model of calcium dynamics in
cardiac cells. This model was originally developed by our client, Leighton T. Izu, from the Department of Pharmacology at UC Davis. We
conducted our research in the summer of 2015 at the UMBC REU Site: Interdiciplinary Program in High Performance Computing.
Problem Statement
We are studying the mechanism by which calcium ions (Ca2+) waves propagate through cardiac muscle, or cardiomyocyte, cells in order to show that certain pathological conditions can lead to electrical anomalies and eventually trigger cardiac arrhythmias. Our purpose in pursuing this topic is driven by the fact that heart-related diseases are the leading cause of mortality in the United States. Waves can be induced by many physiological factors, and our goal is to improve a current mathematical model so that it incorporates influential components that lead to overloading of Ca2+ stores in the sarcoplasmic reticulum (SR) and how these impact resulting electrical anomalies in the heart.
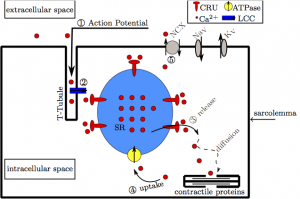
Figure 1. A diagram of the inner mechanisms of the heart cell. The numbers and arrows represent the way calcium flows through the heart cell.
Methodology
Using code developed in C, we incorporated new parameters to simulate the workings of the heart, including the Ca2+ concentration in
the SR, a new SR buffer species (calsequestrin), and voltage. With each of these came necessary manipulation of initial conditions and
the parameters of the mechanisms involved. We examined the SR load to make Ca2+ release from CRU’s dependent on SR concentration,
and also explored the impact of voltage differences across the cell membrane using the Morris-Lecar model. After including these
components, we evaluated data outputs and simulations to observe how these compared to the original model and what these mean within the
overarching scope of Ca2+ release in cardiomyocytes.
Results and Conclusions
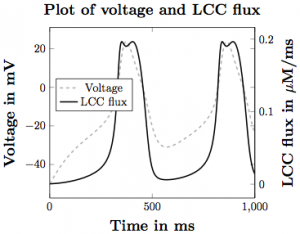
Figure 2. The voltage pulse across the cell membrane overlaid with the calcium flux through the LCC channels.
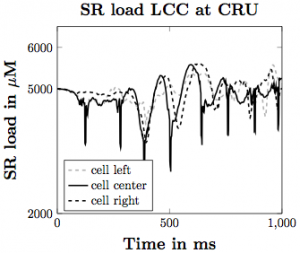
Figure 3. SR calcium load with the voltage model implemented assuming that the LCC channels are directly next to the CRUs.

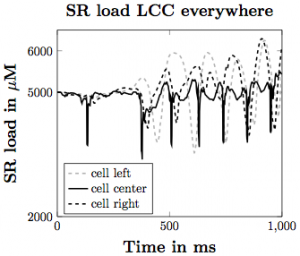
Figure 5. SR calcium load with the voltage model implemented assuming that the LCC channels are everywhere in the cell membrane.

Based on our simulations, we have produced many different findings regarding the influence of the elements we have incorporated on the likelihood of calcium waves in the heart. Increasing SR load leads to more calcium waves, with flooding of Ca2+ in the cell occurring with higher SR Ca2+ diffusion coefficient and stronger CRU probability of release sensitivity. Incorporating SR buffers into the model makes waves less likely to occur under the same parameter set. With the voltage model incorporation, increasing depolarization across the plasma membrane leads to increased Ca2+ influx into the intracellular space of the cell, triggering greater SR Ca2+ release, making waves more likely to occur. These give us a greater understanding about how Ca2+ influences the inner workings of the heart and can lead to cardiac issues in the general population.
Links
Amanda M. Alexander, Erin K. DeNardo, Eric Frazier III, Michael McCauley, Nicholas Rojina, Zana Coulibaly, Bradford E. Peercy, and Leighton T. Izu. Impact of calcium store overload on electrical dynamics of cardiac myocytes. Technical Report HPCF-2015-25, UMBC High Performance Computing Facility, University of Maryland, Baltimore County, 2015. (HPCF machines used: maya.). Reprint in HPCF publications list
Poster presented at the Summer Undergraduate Research Fest (SURF)
Click here to view Team 1’s project
Click here to view Team 2’s project
Click here to view Team 3’s project
Click here to view Team 4’s project
Click here to view Team 6’s project
Click here to view Team 7’s project
Click here to view Team 8’s project